Fusel oil, also known as fuselage or fusel alcohols, is a mixture of higher alcohols, primarily consisting of compounds such as propanol, butanol, and amyl alcohols. It is a byproduct of alcoholic fermentation in the production of alcoholic beverages, particularly during the fermentation of grains like corn, barley, or wheat. Fusel oil is undesirable in many cases due to its strong, unpleasant odor and taste, making it necessary to separate and remove from the final product. The separation process of fusel oil involves several steps aimed at isolating the alcohol compounds for various purposes or eliminating them to enhance the quality of the final product.
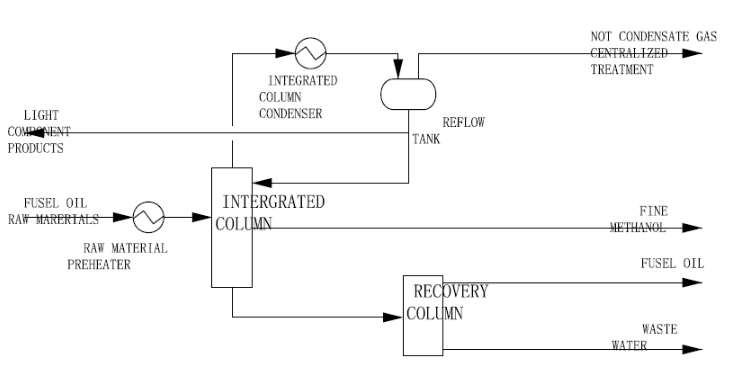
Separation Techniques for Fusel Oil
1. Distillation
Fractional Distillation:
The primary method used for separating fusel oil from alcoholic beverages is fractional distillation. This process takes advantage of the different boiling points of alcohol compounds in the mixture. As fusel oil consists of higher alcohols that have higher boiling points than ethanol, the distillation process can effectively separate them.
During fractional distillation, the fermented mixture is heated in a distillation column. The heat causes the alcohol compounds to vaporize at different temperatures, allowing for the separation of ethanol (which has a lower boiling point) from the higher boiling point fusel alcohols. These higher alcohols are collected separately as fusel oil.
2. Rectification
Rectification Columns:
Rectification involves a continuous distillation process using rectification columns to further purify the fusel oil. Rectification columns consist of trays or plates that allow for multiple vaporization and condensation cycles. This helps separate and concentrate the specific alcohol compounds present in fusel oil, leading to the isolation of individual alcohols like propanol, butanol, and amyl alcohols.
3. Solvent Extraction
Liquid-Liquid Extraction:
Solvent extraction techniques are employed to selectively extract and separate specific compounds from fusel oil. For instance, using a suitable solvent like diethyl ether or other organic solvents, it is possible to extract certain alcohols from the fusel oil mixture, leaving behind unwanted compounds.
4. Adsorption and Chromatography
Adsorption Processes:
Adsorption techniques involve using materials like activated charcoal or zeolites to adsorb specific components of the fusel oil mixture. This method helps in removing impurities or specific alcohols by selectively trapping them on the surface of the adsorbent material.
Chromatography:
Chromatography techniques, such as gas chromatography or liquid chromatography, can be employed for the separation of individual alcohol compounds present in fusel oil. These methods involve passing the fusel oil mixture through a stationary phase, which selectively interacts with different compounds, resulting in their separation based on their affinity for the stationary phase.


Fusel Oil Separation Technology
Applications and Uses of Separated Fusel Oil Compounds
1. Chemical Industry
Butanol Production:
One of the major uses of separated fusel oil compounds, especially butanol, is in the chemical industry. Butanol is utilized in the production of plastics, synthetic rubbers, solvents, and various industrial chemicals.
2. Fuel Additive
Biofuels:
Some of the separated alcohols from fusel oil, particularly ethanol, can be used as biofuel additives. Ethanol, for instance, is blended with gasoline in varying proportions to produce ethanol-blended fuels, which are considered more environmentally friendly and renewable.
3. Perfumes and Flavors
Aromatic Compounds:
Certain alcohols extracted from fusel oil are used in the fragrance and flavor industry to create specific aromas and flavors in perfumes, cosmetics, and food products.
4. Pharmaceuticals
Pharmaceutical Intermediates:
Isolated alcohol compounds from fusel oil can serve as intermediates in the production of pharmaceuticals and medicinal compounds due to their chemical properties.
Challenges in Fusel Oil Separation
Complexity of the Mixture
Fusel oil is a complex mixture containing multiple alcohol compounds, which makes its separation challenging. Differentiating and isolating individual alcohols require precise and often energy-intensive separation processes.
Purity and Efficiency
Achieving high purity levels of separated alcohol compounds while maintaining efficiency in the separation process remains a challenge. Impurities or remaining traces of other compounds can affect the quality and usability of the separated alcohols.
Economic Viability
The cost-effectiveness of separating fusel oil compounds is a consideration, especially when the demand for specific alcohols might not justify the expenses involved in their separation and purification.
Conclusion
The separation of fusel oil is a crucial process in various industries, aiming to extract and isolate specific alcohol compounds for diverse applications. Techniques like distillation, rectification, solvent extraction, adsorption, and chromatography play key roles in achieving the separation of fusel oil compounds. Despite the challenges posed by the complexity of the mixture and the need for high purity, the separated alcohols find applications in industries ranging from chemical production to pharmaceuticals, biofuels, flavors, and fragrances. Continued research and advancements in separation technologies may further enhance the efficiency and economic viability of isolating fusel oil compounds for various industrial applications.


















