Mono ethylene glycol (MEG), also known simply as ethylene glycol, might sound appealing, but don't be fooled! This widely used industrial chemical is a colorless, odorless liquid with diverse applications. From keeping your car's engine from freezing in winter to creating the soft fleece in your favorite jacket, MEG is involved in numerous aspects of daily life. But is MEG safe? How is it produced, and what exactly are its various applications? In this blog post, we’ll dive into the world of mono ethylene glycol, separating fact from fiction and uncovering the science behind this remarkable chemical.
Introduction to MEG
Mono ethylene glycol (MEG), also known as EG, 1,2-ethanediol, or 1,2-dihydroxyethane, is a clear, colorless liquid with a slightly syrupy consistency. Despite its slightly sweet taste, it has almost no odor. MEG is a highly versatile compound that mixes well with water, alcohols, and many organic substances. Its most prominent uses are in the production of polyester fibers for textiles and as a key component in antifreeze, coolants, and aircraft de-icing fluids.
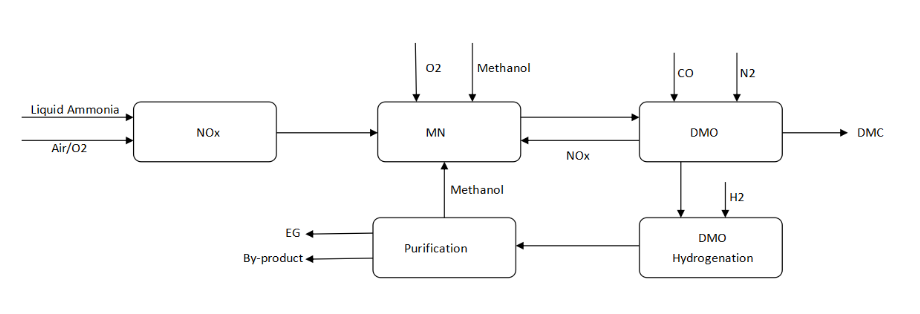
Manufacturing Process
The manufacturing process of Mono Ethylene Glycol (MEG), an important industrial chemical used in the production of polyester fibers, resins, and antifreeze, primarily involves the oxidation of ethylene followed by hydration. Here’s a breakdown of the typical process:
1. Ethylene Oxidation to Ethylene Oxide
- Raw Material: Ethylene gas is the main feedstock.
- Catalytic Oxidation: Ethylene is reacted with oxygen in the presence of a silver oxide catalyst at high temperatures (200-300°C) and pressures (10-30 atm).
- Reaction: Ethylene (C₂H₄) is oxidized to produce ethylene oxide (C₂H₄O).
- Control: Reaction conditions are carefully controlled to prevent over-oxidation, which would produce carbon dioxide and water.
Reaction:
C2H4+½O2→C2H4O
2. Ethylene Oxide Hydration to Mono Ethylene Glycol
- Direct Hydration: Ethylene oxide is reacted with water to produce mono ethylene glycol (MEG) and byproducts like diethylene glycol (DEG) and triethylene glycol (TEG).
- Catalyst: This hydration reaction can be either acid- or base-catalyzed, but typically uses an acidic environment.
- Conditions: The hydration reaction occurs at moderate temperatures (50-70°C) and pressures (1-3 atm).
Reaction:
C2H4O+H2O→HOCH2CH2OH
3. Separation and Purification
- Distillation: The reaction mixture contains MEG, DEG, TEG, and unreacted ethylene oxide. This mixture is separated using distillation columns to purify MEG.
- Byproduct Management: DEG and TEG are separated and often sold or used in other industrial applications.
4. Product Quality Control
- Quality Testing: MEG undergoes quality testing to ensure it meets specifications for intended applications, such as polyester production or antifreeze formulations.
- Packaging: Purified MEG is then cooled, packaged, and stored for distribution.
The production of MEG is energy-intensive and must be carefully managed to control environmental impact and maximize yield. Some plants are now also adopting more sustainable ethylene sources, such as bio-based ethylene, to produce bio-MEG, reducing reliance on petrochemical feedstocks.

Major Applications of Mono Ethylene Glycol (MEG)
1. Chemical Intermediates
MEG is essential for producing Polyethylene Terephthalate (PET), widely used in manufacturing polyester films and PET bottles for food and beverage packaging. These PET products are fully recyclable and certified safe for food and beverages. MEG’s purity directly affects PET quality, and MEG produced via gas chromatography (GC) achieves an outstanding purity of 99.9% by weight, ensuring the creation of premium PET materials.
2. Polyester Fibers
MEG is a critical building block in polyester fiber production, extensively used in clothing and textiles. High-purity MEG, such as GC Glycol’s 99.9% pure product, is essential for producing high-quality polyester fibers known for their durability, comfort, wrinkle resistance, and easy maintenance. Polyester fibers can also be blended with other fibers to enhance specific properties.
3. Coolants
MEG is a key component in engine coolant formulations. It raises the boiling point of the water mixture, enhancing the coolant’s ability to regulate engine temperature. This functionality protects the engine from overheating in hot weather and freezing in cold climates, ensuring optimal performance and longevity.
4. De-icing and Anti-freeze
In industrial applications, MEG is widely used in antifreeze formulations due to its excellent ability to lower water’s freezing point. This property makes it invaluable in coolants, aircraft anti-icers, and deicers, providing effective performance and protection in freezing conditions.
Market Outlook
The Mono Ethylene Glycol (MEG) market continues to grow, fueled by its versatility and high demand across numerous industries. Known for its durable, hydrophobic, and resilient properties, MEG is essential in producing polyester fibers, polyester films, polyethylene terephthalate (PET), antifreeze products, coolants, solvents, and more. Its humectant quality makes it valuable in paper, printing inks, leather, fiber treatments, and cellophane manufacturing. MEG plays a crucial role across various end-use sectors, including textiles, packaging, cosmetics, pharmaceuticals, food and beverage, automotive, chemicals, adhesives, and sealants. Globally, most MEG production supports polyester fiber manufacturing, followed closely by PET and polyester films, reinforcing its status as a cornerstone of industrial chemistry that drives innovation and market growth.
Conclusion
In summary, the Mono Ethylene Glycol (MEG) market is expected to expand due to its versatility and wide-ranging applications. MEG’s key attributes—durability, hydrophobicity, and tenacity—make it indispensable in manufacturing polyester fibers, films, PET, antifreeze, coolants, and solvents. Its humectant nature further supports its use in industries like paper, printing inks, leather, and fiber treatments. As an essential feedstock, MEG is integral to various sectors, including textiles, packaging, cosmetics, pharmaceuticals, and more. MEG’s importance in global demand highlights its role in driving innovation and growth across industries. The MEG market remains adaptable, poised to meet future challenges and seize opportunities in evolving markets.

















